Sleep Profiler PSG2
Manufacturer: ABM
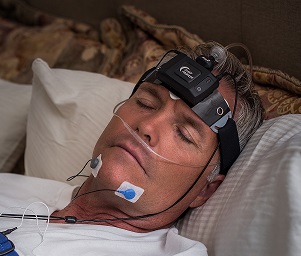
The Sleep Profiler PSG2 is a lightweight device includes voice messages and other intuitive design features that enable reliable self-application in the home with acquisition across multiple nights. The Sleep Profiler PSG2 is the only home sleep apnoea test that uses voice messages to assist the patient to correctly self-apply the sensors.
The Sleep Profiler PSG2 is a lightweight device includes voice messages and other intuitive design features that enable reliable self-application in the home with acquisition across multiple nights. The Sleep Profiler PSG2 is the only home sleep apnoea test that uses voice messages to assist the patient to correctly self-apply the sensors.
The Sleep Profiler PSG2 can be used to detect sleep-disordered breathing such as:
- Detection of apnoeas, hypopnea-desats, and hypopnea-arousals
- Differentiation between obstructive and central apneas
- Hypoxemic exposure during sleep
- REM and positional influence on OSA severity
- Frequency and intensity of positional snoring
- Power spectral characteristics of the sleep stages.
The 13-channel system includes EEG, EOG and EMG, wireless oximetry, nasal pressure/airflow, chest and abdomen respiratory effort (RIP or piezo), forehead and finger pulse rate, head movement and position, and quantitative snoring. Sleep Profiler PSG2 is ideal for multi-night or repeated measures assessment.
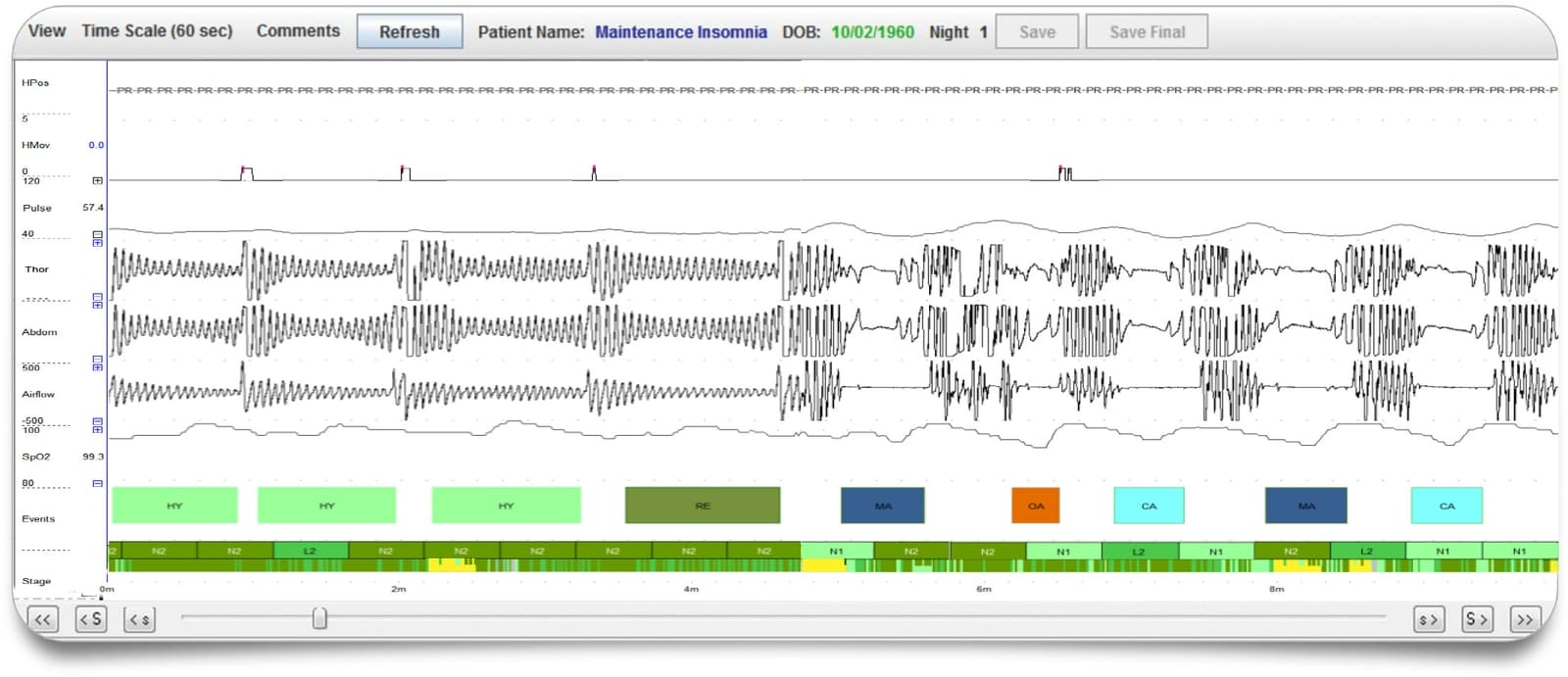
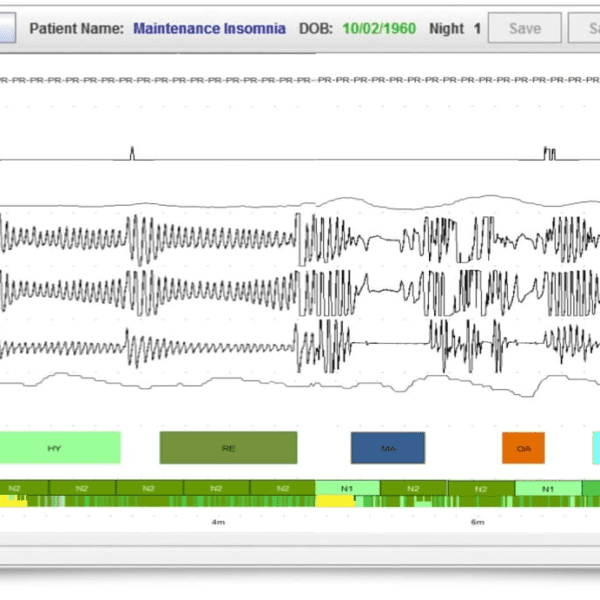
Sleep Profiler PSG2 combines web-based presentation of recorded signals with validated auto-staging and technical editing capabilities to significantly reduce scoring time. The web-based software enables selection of either 3% or 4% desaturation criteria, with auto-scored apnoea/hypopnea indexes based on both AASM²⁰¹² and AASM²⁰⁰⁷ scoring rules and automated detection of poor signal quality. Interpretation features include customised editing, touchpoint insertion of key diagnostic and treatment recommendations, and secure signature insertion for quick and easy reporting.
Sleep Profiler PSG2 Features:
- Records EEG, EOG, EMG, with optional sub-mental EMG or ECG, pulse rate, head position, head movement, quantitative snoring
- Easy application and voice prompts to ensure patients can reliably self-apply at home
- Auto-staging and technical editing capabilities to significantly reduce scoring time. Auto-staging accuracy equivalent to PSG-based manual staging variability with:
- Detection of apnoeas, hypopnea-desats, and hypopnea-arousals
- Differentiation between obstructive and central apneas
- Sleep spindles, spindle duration and power spectral densities
- Movement arousals, cortical arousals, microarousals and autonomic activations
- Sleep latency, REM latency, N3 latency
- The software monitors study status and automatically sends reminder emails so studies are not overlooked
- Ability to do multi-night studies with night-to-night comparisons, with up to 30 hours of in-home recording between battery charges
- Available with application of novel sleep biomarkers
- Consumable kits are purchased in quantities of 50, and are available with the option of human scoring review
- TGA registered.
Clinical
PSG2 enables clinicians to easily and accurately conduct unattended PSG studies to:
- Rule out potential false negative Type 3 study results
- Provide PSG studies to patients who are:
- In need based on co-morbidities, but unable to schedule a lab-based study
- Unable to sleep during a laboratory PSG
- Suspected of upper airway resistance syndrome
- Suspected of having neurodegenerative disease.
Research
PSG2 provides researcher the capability to assess the interaction between sleep architecture, hypoxemia, and sleeping position on sleep disordered breathing severity with variables that include:
- REM and Non-REM apnea/hypopnea, apnoea, and hypopnea indexes
- Snoring above 30, 40, 50, and 60 dB by position
- Number of hypopneas confirmed by arousals vs. desaturations by position
- Number of apnoeas and hypopnea events by position
- 3% and 4% oxygen desaturation indexes by position.
Reduced non–rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease.
Head Position During Sleep: Potential Implications for Patients with Neurodegenerative Disease
The Need for a Reliable Sleep EEG Biomarker
Multimodal Assessment of Sleep in Men and Women During Treatment for Opioid Use Disorder
Send us a message about this product