About
Introducing the Sleep Profiler PSG2 by Advanced Brain Monitoring – the cutting-edge solution for both clinical and research demands in sleep studies. Designed with versatility in mind, the Sleep Profiler PSG2 seamlessly caters to level 2 and 3 sleep studies, offering unparalleled insights into sleep patterns and disorders. Its advanced monitoring capabilities extend beyond traditional sleep analysis, making it an indispensable tool for diagnosing neurodegeneration and detecting ICU delirium and sepsis in hospital settings.
With its comprehensive features, robust scoring algorithms and precise data collection, the Sleep Profiler PSG2 stands at the forefront of innovation, empowering healthcare professionals with invaluable insights into patient care and research endeavours.
SYSTEM REVIEWApplications
Clinical:
PSG2 enables clinicians to easily and accurately conduct unattended PSG studies to:
- Rule out potential false negative Type III study results
- Provide PSG studies to patients who are:
- hospitalised and need to qualify for CAP reimbursement
- in need based on co-morbidities, but unable to schedule a lab-based study
- unable to sleep durina a laboratory PSG
- suspected of upper airway resistance syndrome
- suspected of having neurodegenerative disease.
Research:
PSG2 provides researcher the capability to assess the interaction between sleep architecture, hypoxemia, and sleeping position on sleep disordered breathing severity with variables that include:
- REM and Non-REM apnea/hypopnea, apnea, and hypopnea indexes
- Snoring above 30, 40, 50, and 60 dB by position
- Number of hypopneas confirmed by arousals vs. desaturations by position
- Number of apneas and hypopned events by position
- 3% and 4% oxygen desaturation indexes by position.
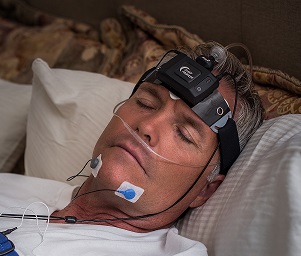
The Sleep Profiler PSG2 combines the accuracy and versatility of the Sleep Profiler with the cardio-respiratory signals needed for a Type 2 unattended polysomnography study. This lightweight device includes voice messages and other intuitive design features that enable reliable self-application in the home with acquisition across multiple nights.
The Sleep Profiler PSG2 can be used to detect sleep-disordered breathing such as:
- Detection of apnoeas, hypopnea-desats, and hypopnea-arousals
- Differentiation between obstructive and central apneas
- Hypoxemic exposure during sleep
- REM and positional influence on OSA severity
- Frequency and intensity of positional snoring
- Power spectral characteristics of the sleep stages.
The 13-channel system includes EEG, EOG and EMG, wireless oximetry, nasal pressure/airflow, chest and abdomen respiratory effort (RIP or piezo), forehead and finger pulse rate, head movement and position, and quantitative snoring. Sleep Profiler PSG2 is ideal for multi-night or repeated measures assessment.
Sleep Profiler PSG2 combines web-based presentation of recorded signals with validated auto-staging and technical editing capabilities to significantly reduce scoring time. The web-based software enables selection of either 3% or 4% desaturation criteria, with auto-scored apnoea/hypopnea indexes based on both AASM²⁰¹² and AASM²⁰⁰⁷ scoring rules and automated detection of poor signal quality. Interpretation features include customised editing, touchpoint insertion of key diagnostic and treatment recommendations, and secure signature insertion for quick and easy reporting.
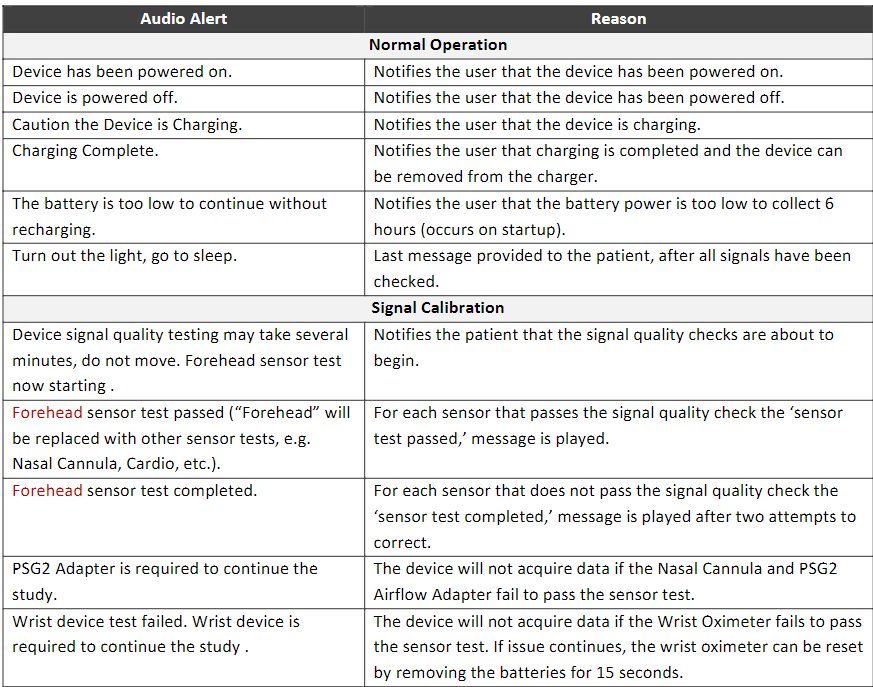
The Sleep Profiler PSG2 is the only home sleep apnoea test that uses voice messages to assist the patient to correctly self-apply the sensors. Real-time detection of poor airflow and oximetry signal quality trigger corrective voice alerts that contribute to an industry-leading 94% success rate for a Type 2 study.
Specifications:
- Channels – Up to 13
- Power – Rechargeable Battery
- Warranty – 2 years
- Weight, including WristOx® and Effort Belts – up to 388g (13.7oz)
- Memory – 8 GB internal
- Recording Time – Up to 30 hours
- Airflow range – ‡ 2cm H2O
- Oximetry accuracy – ‡ 2% at one standard deviation from 70% to 100%.
Applications
Clinical:
Sleep Profiler enables clinicians to easily and accurately:
- Differentiate short sleep syndrome from sleep state misperception
- Identify abnormal sleep patterns attributed to chronic or neurodegenerative diseases
- Monitor the effects of OSA therapy on sleep quality
- Assess the dose-dependent medication effects on sleep architecture and sleep continuity.
Sleep Profiler is ideal for multi-night or repeated measures assessment of patients complaining of symptoms consistent with:
- Insomnia
- Hypersomnia
- Other non-OSA sleep disorders.
Research:
Sleep Profiler is being used by researchers to assess:
- REM sleep without Atonia in patients with RBD
- Prodromal sleep biomarkers for neurodegenerative disease
- Effect of Suvorexant progressive supranuclear palsy
- Differences between normal cognition and mild cognitive impairment, Alzheimer’s disease, Parkinson’s Dementia, and
- Dementia with Lewy Body
- Sleep patterns in patients with ICU delirium
- Effects of medication type, timing, and dosage on ICU sleep and outcomes
- Sleep quality changes as a result of cannabis and opioid dependency
- Outcomes resulting from an insomnia intervention
- Influence of sleep deprivation on memory.
Sleep Profiler™ EEG Sleep Monitor bridges the gap between laboratory polysomnography and actigraphy. Three frontopolar channels provide the EEG, EOG and EMG signal features needed to characterise by-stage sleep time. This lightweight, wireless device includes voice messages and other intuitive design features that enable reliable self-application in the home with acquisition across multiple nights.
Sleep Profiler is ideal for multi-night or repeated measures assessment of patients complaining of symptoms consistent with:
- Insomnia
- Hypersomnia
- Other non-OSA sleep disorders.
Sleep Profiler combines web-based presentation of recorded signals with validated auto-staging and technical editing capabilities to significantly reduce scoring time. Interpretation features include customised editing, touchpoint insertion of key diagnostic and treatment recommendations, and secure signature insertion for quick and easy reporting.
Report formats include automated generation of medical history summaries, medication usage, comparison to age/sex normative ranges, and patient reports that contrast their sleep diary entries to the objective sleep quality measures.
The new Sleep Profiler RTA software combines wireless transmission of the signals to a tablet with real-time sleep staging for the monitoring of sleep quality and abnormal EEG patterns in hospitalised patients or in research applications.
Specifications:
- Channels – Up to 8
- Power – Rechargeable Battery
- Warranty – 2 years
- Dimensions – 7cm x 4.5cm x 1.75cm
- Weight, Including Batteries – 71g (2.5oz)
- Memory – 8 GB internal
- Recording Time – Up to 30 hours.